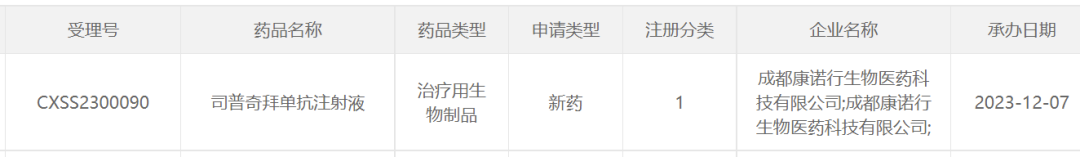
Important Notice
Recently, it has come to our attention that certain individuals that have impersonated 3H to publish fake and misleading information and carry out illegal activities such as financing and capital raising via websites, apps and other channels.
3H hereby declares:
1.“三正健康”, “三正健康投资”, “3H HEALTH”, “3H”, “3h partner”, “3h health”are all legally registered by our company (including our affiliated parties), and shall not be used illegally by anyone without our permission.
2.3H official contact information is as follows. If you have any further questions, please visit our official website or call +86 21 6107 2498
a.Official Website: www.3hhinvestment.com;
b.Official WeChat Account: 3H Health Investment (WeChat ID: 三正健康投资)
c.Official LinkedIn Account: https://www.linkedin.com/company/3h-health-investment-management/
3.3H Health Investment has never authorized and will never authorize any entity or individual to conduct any public fundraising activities in the name of 3H Health through any channels such as websites, apps, WeChat, and Weibo.
All individuals and groups participating in the above activities are involved in illegal capital raising. We disclaim any legal or commercial relationships thereof. These illegal activities have seriously damaged 3H’s reputation, and we reserve the right to take legal actions against the above-mentioned entities and individuals. Please stay alert to false information to avoid any financial loss.